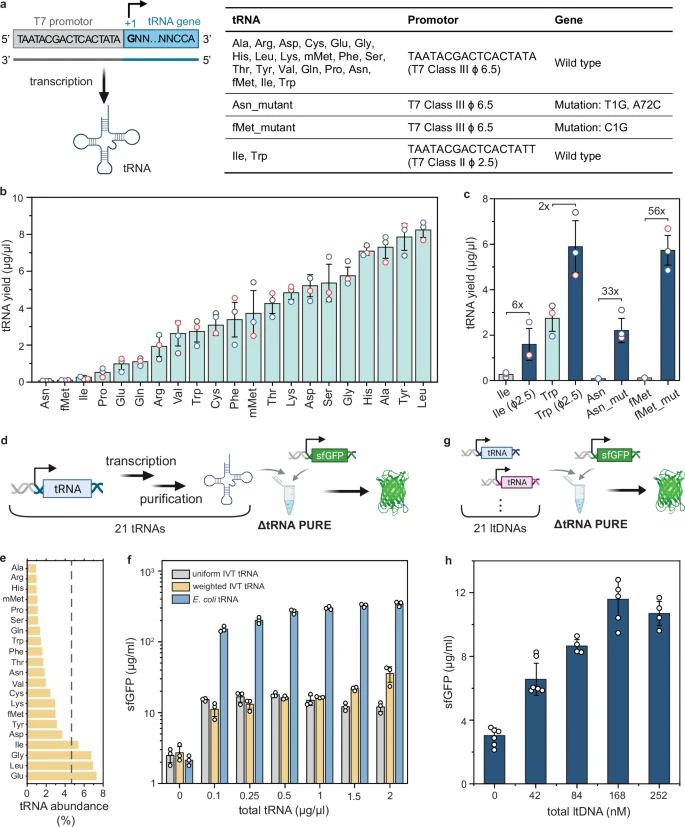
|
| Role of miR-2954 in male chicken development (Source: Fallahshahroudi et al., Nature, 2025) |
Have you ever wondered how different sexes in the animal kingdom manage their genes, especially when their sex chromosomes are so varied? In birds, it's a fascinating story! Unlike mammals where males are XY and females XX, birds have a ZW system where females are ZW and males are ZZ. This means female birds have only one copy of many Z-linked genes, while males have two. This difference in gene "dosage" needs to be compensated for, and new research reveals a surprising hero in this process: a tiny molecule called miR-2954.
A groundbreaking study published in Nature (2025) by Amir Fallahshahroudi and an international team of researchers has uncovered that this male-essential microRNA is a key player in avian sex chromosome dosage compensation. Let's dive into what they found!
The Challenge of Dosage Compensation in Birds
Over evolutionary time, the W chromosome in female birds has lost most of its genes. This poses a challenge: how do females, with only one Z chromosome, ensure they have enough of the gene products from their Z-linked genes, especially when males have two Z chromosomes? This is called dosage compensation.
Previous research suggested that the expression of Z-linked genes is generally higher in males than in females. However, the exact mechanisms and the extent of this compensation have been unclear. That's where miR-2954 comes in.
miR-2954: A Male-Specific Guardian
The researchers honed in on miR-2954, a microRNA (a small non-coding RNA molecule that regulates gene expression) located on the Z chromosome. What's special about miR-2954? It's predominantly expressed in males (5 to 10 times higher than in females!), and its predicted targets are mostly Z-linked genes that are considered "dosage-sensitive" – meaning their expression levels need to be tightly controlled. This hinted at a crucial role for miR-2954 in male birds.
Knocking Out miR-2954: A Lethal Consequence for Males
To understand miR-2954's function, the scientists performed a knockout (KO) experiment in chickens using advanced CRISPR-Cas9 genome editing. They generated chickens where the miR-2954 gene was deleted. The results were striking:
Homozygous knockout males (ZKOZKO) died early in embryonic development, specifically before embryonic day 7 (E7). This indicates that miR-2954 is essential for male survival.
Female knockouts (ZKOW) and heterozygous males (ZKOZ) showed no adverse effects and survived normally. This aligns with the low expression of miR-2954 in females and suggests that one functional copy of the gene is sufficient in males.
Unraveling the Mechanism: Gene Upregulation in KOs
So, why was the loss of miR-2954 lethal for male embryos? The researchers investigated gene expression changes in the knockout embryos. They found that in homozygous male KOs, Z-linked genes predicted to be targets of miR-2954 were significantly upregulated (their expression levels increased). This upregulation wasn't just at the transcriptional level (mRNA), but also at the translational level (protein synthesis).
This makes sense! MicroRNAs typically work by degrading target mRNA, thereby reducing protein production. When miR-2954 is absent, its target mRNAs are no longer degraded, leading to an overabundance of these gene products.
Furthermore, they discovered that these upregulated Z-linked target genes are dosage-sensitive and play critical roles in development. Many of them are "ohnologues," genes retained from ancient whole-genome duplications and are often intolerant to changes in dosage. Their widespread expression across tissues and vital developmental functions explain the severe and lethal phenotypes observed in the male knockouts, including delayed growth and abnormalities in organs.
An Evolutionary Tale of Compensation
The study proposes a compelling evolutionary model for avian dosage compensation:
W Chromosome Decay: As the W chromosome lost genes over time, female birds faced a dosage reduction for Z-linked genes.
Female & Male Upregulation: To compensate, females evolved mechanisms for transcriptional and translational upregulation of dosage-sensitive Z-linked genes on their single Z chromosome, aiming to restore ancestral expression levels. This upregulation also occurred in males.
Male-Specific Repression by miR-2954: In males, with two Z chromosomes, this upregulation led to an excess of Z-linked gene products. To counteract this, miR-2954 emerged in the avian lineage and evolved to specifically degrade these excess transcripts in males.
This elegant system ensures that despite having different numbers of Z chromosomes, dosage-sensitive Z-linked genes achieve balanced expression levels between male and female birds.
A Unique Dosage Compensation System
This research highlights a unique role for a microRNA in dosage compensation, setting it apart from other known systems. For instance, in placental mammals, X-chromosome inactivation (mediated by lncRNAs like XIST) compensates for the two X chromosomes in females. In contrast, birds utilize a targeted post-transcriptional repression by miR-2954 to manage gene dosage in males.
This study fundamentally changes our understanding of sex chromosome evolution and dosage compensation in birds. It unveils miR-2954 as a critical regulator that sculpts the male transcriptome and ensures their survival. This tiny molecule truly plays a mighty role!
What do you find most surprising about this avian dosage compensation system compared to what's known in mammals?
Original Article: Fallahshahroudi, A., Yousefi Taemeh, S., Rodríguez-Montes, L. et al. A male-essential miRNA is key for avian sex chromosome dosage compensation. Nature (2025). https://doi.org/10.1038/s41586-025-09256-9
Author: KuriousK. | Subscribe: Don’t miss updates—follow this blog!