 |
Image from Mahendran et al. Nat. Chem. (2025). |
Imagine peering deep inside a brain cell, but something is terribly wrong. Instead of a smoothly running system, you find tiny, toxic knots of genetic material called RNA. These solid, stubborn clumps act like sponges, soaking up essential proteins the cell needs to survive. This cellular sabotage is a hallmark of devastating neurological diseases like Huntington's and ALS, and for a long time, these clumps were considered irreversible.
One of the biggest mysteries in neurodegenerative disease research has been: how do these harmful clusters form in the first place?
Now, in a groundbreaking study published in Nature Chemistry, researchers at the University at Buffalo have not only answered that question but have also demonstrated a stunning way to untie these knots, preventing and even disassembling them.
The Cellular Crime Scene: How Good Droplets Go Bad
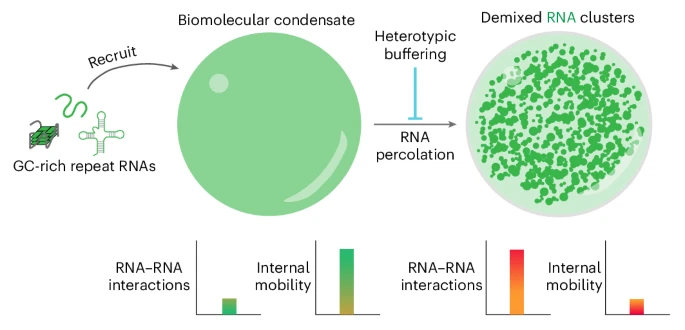
The secret, the researchers discovered, lies within tiny, liquid-like droplets in our cells known as biomolecular condensates. Think of them as the cell's pop-up meeting rooms, where proteins and nucleic acids (like RNA) gather to get work done.
The culprits are specific, disease-linked RNA molecules with long, repetitive sequences. These "repeat RNAs" are inherently sticky.
"They don't stick to each other just by themselves," explains Tharun Selvam Mahendran, the study's first author. "They need the right environment to unfold and clump together, and the condensates provide that."
Inside these droplets, the sticky RNAs begin to glom onto each other, forming a dense, solid core. Alarmingly, the team found that these solid clusters remain even after the host droplet dissolves. "This persistence," Mahendran adds, "is partly why the clusters are thought to be irreversible."
The Heroes Emerge: A Two-Pronged Attack on Disease
Having identified the crime scene, the team, led by Dr. Priya Banerjee, an associate professor of physics at UB, engineered a brilliant two-pronged solution: one to prevent the clumps and another to break them apart.
1. The Molecular Chaperone (Prevention):
First, they found that a naturally occurring protein called G3Bp1 could act as a bodyguard. When introduced into the mix, G3Bp1 latches onto the sticky RNA strands.
"The RNA clusters come about from the RNA strands sticking together, but if you introduce another sticky element... then the interactions between the RNAs are frustrated and clusters stop forming," Dr. Banerjee explains. "You can think of the G3BP1 as an observant molecular chaperone that binds to the sticky RNA molecules and makes sure that RNAs don't stick to each other."
2. The Disassembly Crew (Reversal):
But what about the clumps that have already formed? For this, the team designed a powerful tool: an antisense oligonucleotide (ASO). An ASO is a small, engineered strand of RNA designed to be a perfect mirror image of the problematic repeat RNA.
When this ASO is introduced, it seeks out the clumped RNA, binds to it like a key fitting a lock, and pulls the toxic cluster apart. The result is dramatic.
"It's fascinating to watch these clusters form over time... under the microscope," says Banerjee. "Just as striking, the clusters dissolve when antisense oligonucleotides pull the RNA aggregates apart."
The team's engineered strand of RNA (the ASO) was so precise that if its sequence was scrambled even slightly, it failed to work. This specificity is a huge advantage. "This suggests our ASO can be tailored to only target specific repeat RNAs, which is a good sign for its viability as a potential therapeutic application," Banerjee notes.
A New Horizon for Neurological Disease
This discovery cracks open a new window of understanding into how diseases like ALS and Huntington's progress at a molecular level. More importantly, it provides a tangible strategy and a powerful tool for fighting back. By demonstrating that these "irreversible" clumps can, in fact, be dissolved with precision-engineered molecules, the University at Buffalo team has ignited a new beacon of hope for developing future treatments for these devastating conditions.
It’s a powerful reminder of the dual nature of our own biology, where the same fundamental molecules—in this case, RNA—can hold the secrets to both the origins of life and the keys to conquering disease.
Source: Mahendran, T.S., Wadsworth, G.M., Singh, A. et al. Homotypic RNA clustering accompanies a liquid-to-solid transition inside the core of multi-component biomolecular condensates. Nat. Chem. (2025). https://doi.org/10.1038/s41557-025-01847-3
Author: KuriousK. | Subscribe: Don’t miss updates—follow this blog!
No comments:
Post a Comment