 |
Image source: Li et al., Nat Commun 16, 6212 (2025) |
What if you could build a biological machine that could create its own parts and run indefinitely? It sounds like science fiction, but researchers are taking a major step towards this incredible goal. In a new study published in Nature Communications, scientists have successfully built a system that can continuously create all the components it needs to produce proteins, the workhorses of all living things.
This breakthrough is a huge leap forward in the field of synthetic biology, with the potential to revolutionize everything from medicine to manufacturing.
The Challenge: Building a "Perpetual Motion" Machine for Biology
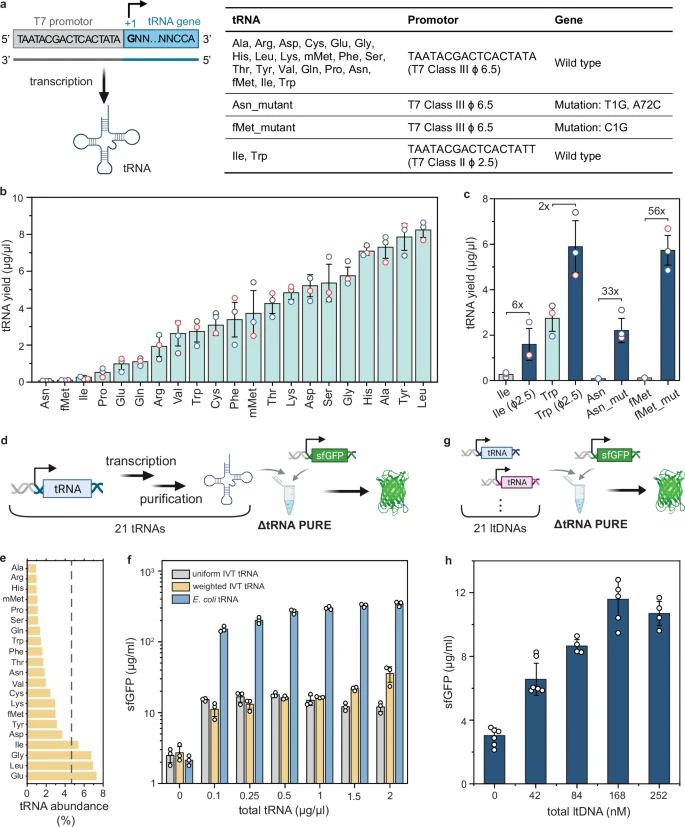
At the heart of all life is the process of translation – the way cells read genetic code (in the form of messenger RNA) and build proteins. This process requires a whole toolkit of molecules, including ribosomes (the protein-building factories) and transfer RNAs (tRNAs), which act as the delivery trucks, bringing the right amino acid building blocks to the ribosome.
Scientists have been working with "cell-free" systems, essentially a bag of these molecular parts, to produce proteins outside of a living cell. One of the most advanced is the PURE system, which contains all the necessary components for protein synthesis. However, a major limitation has been that the tRNAs in the system eventually get used up, and the protein production grinds to a halt.
The ultimate goal is to create a self-regenerating system, one that can not only produce proteins but also create the very tRNAs it needs to keep the process going. This has been a significant challenge.
The Breakthrough: A System That Builds Itself
The research team tackled this problem head-on. Their solution was to create a system that could synthesize its own tRNAs in situ, meaning right within the reaction mixture.
Here’s how they did it:
- Creating a Complete Set of tRNAs: First, the team had to create a full set of 21 different tRNAs that could be transcribed (or read from a DNA template) and then used for protein synthesis. They found that by adjusting the amounts of each tRNA, they could significantly improve the protein yield.
- In Situ Synthesis: Next, they showed that they could produce proteins by adding the DNA templates for the tRNAs directly into the PURE system. The system would then transcribe these templates to create the tRNAs it needed, which would then be used to produce the desired protein.
- Continuous Production: The most exciting part of the study came when the researchers put their system on a microfluidic chemostat. This device allowed them to continuously feed the system with fresh nutrients and remove waste products. The result? The system was able to continuously produce its own tRNAs and, in turn, sustain a steady level of protein production over a long period.
Why This Is a Breakthrough
This research is a critical step towards creating a truly synthetic cell, a self-replicating, self-sustaining biological machine. While that is still a long way off, the implications of this work are vast:
- On-Demand Drug and Vaccine Production: Imagine being able to produce medicines and vaccines quickly and on-demand, anywhere in the world, without the need for complex and expensive cell cultures.
- Advanced Materials: This technology could be used to create new biomaterials with unique properties.
- A Deeper Understanding of Life: By building a biological system from the ground up, we can gain a much deeper understanding of the fundamental principles of life itself.
This study is a beautiful example of how engineering principles can be applied to biology to create powerful new technologies. The researchers have not only solved a major technical challenge but have also opened the door to a whole new world of possibilities in synthetic biology. The dream of a self-sustaining, protein-producing machine is now one step closer to reality.
Source:
Li, F., Baranwal, A.K. & Maerkl, S.J. Continuous in situ synthesis of a complete set of tRNAs sustains steady-state translation in a recombinant cell-free system. Nat Commun 16, 6212 (2025). https://doi.org/10.1038/s41467-025-61671-8
Author: KuriousK. | Subscribe: Don’t miss updates—follow this blog!
No comments:
Post a Comment